Last week, inspired by some recent schoolwork and research, and mildly prompted by my collapse at Rocky Raccoon, I started a series of posts on distance running and cardiac health. The first post used my last twenty miles at Rocky as a jumping-off point to talk a little bit about pulmonary edema, and rather obliquely about cardiac illness. I'd like to delve a little bit more into the relationship between endurance exercise, heart health, and heart disease. In light of some of the recent media coverage of these issues, we're going to discuss some facts and address some common misconceptions and/or misinterpretations of some of the data out there, with the goal of all of us becoming better informed regarding this topic and better able to make rational decisions about our athletic future.
Before we can get into dysfunction, though, we have to talk about normal function, and about the physiologic adaptations that the heart makes to long-term endurance exercise. Many of these adaptations are beneficial, but they're not without problems, either.
The normal heart
I don't think there's any need to get into a bunch of esoteric facts about the heart (It pumps six liters of blood per minute! It weighs 300 grams!) but we should first go through a few basics. I'm sure you can remember from ninth grade biology that the heart is a muscle that pumps blood through the body. You might also remember that the heart is split into two sides (left and right), each of which has two chambers (an atrium and a ventricle). The right side of the heart pumps de-oxygenated blood to the lungs, where the red blood cells bind to oxygen. Blood from the lungs then returns to the left side of the heart, from where (whence?) it is pumped out to the rest of the body so that the various tissues and organs can use that oxygen. Having delivered oxygen to the tissues, the blood then returns to the right side of the heart to begin the cycle again. Blood flows throughout the circulatory system in what is essentially a series of tubes; veins carry blood to the heart, while arteries carry blood from the heart.
OK, simple enough. From a basic standpoint, that's all we need the heart to do: pump oxygen-poor blood to the lungs, deliver oxygen-rich blood to the rest of the body. So when we talk about cardiac disease, we're most generally talking about a failure of the heart to fulfill that function. But there are a bunch of different ways in which this basic function can be compromised. For our purposes, there are three systems inherent to normal heart function that we want to be familiar with in order to understand possible dysfunction: the coronary arteries, the conduction system, and the heart muscle itself.
We spoke briefly about the heart muscle last week; simply put, the muscle squeezes, increasing the pressure within the chambers of the heart, and forces blood out into the circulation. The muscle is the heart's engine. The coronary arteries are responsible for delivering oxygen to the heart muscle. Wait a minute, you're saying, didn't you just say that arteries carry blood AWAY from the heart? Yes, I did! Thanks for paying attention! Arteries do indeed carry blood away from the ventricles, but in this case they don't have to go very far. The coronary arteries arise from the aorta immediately after the blood leaves the left ventricle, and they surround the heart, supplying oxygen-rich blood to the muscle. When you hear the term "heart attack," this is usually used to mean an interruption of blood flow to the heart muscle, usually due to a narrowing of, or blockage within, the coronary arteries. We're going to do an entire post about the coronary arteries next week, so for now, just think of them as the heart's plumbing system.
The conduction system, then, is the wiring. This system is comprised of electrical fibers that coordinate the heartbeat. The depolarization of these electrical cells causes the atria, and then the ventricles, to contract synchronously. The contraction of the atria forces blood into the ventricles, and the contraction of the ventricles forces blood out into the circulation. When you see that familiar tracing that we all know represents a beating heart:
what you're looking at is a graphic representation of the heart's electrical activity. (I'm not going to go into what each of those little squiggles means, but if you're interested, read this.) Without the orderly input of the electrical/conduction system, these contractions may lose their synchronicity, robbing the heart muscle of its ability to pump blood effectively--or contractions can cease altogether.
The athlete's heart
Like any other muscle, the heart responds to exercise by adapting to stress. Weight lifting, for example, places the skeletal muscles under stress, ultimately causing the muscles to adapt by increasing muscle mass and size (hypertrophy). Similarly, aerobic exercise means that the muscles requires more oxygen, necessitating increased cardiac output (the amount of blood the heart pumps). Over time, the heart muscle adapts by increasing the mass and thickness of the muscular wall of the left ventricle. Other adaptations include dilation (or enlargement) of the various heart chambers, and dilation of the coronary arteries (which I'll discuss more in next week's post). In the absence of a history of vigorous exercise, many of these structural changes--hypertrophic ventricular walls, atrial dilation--would be considered pathologic. That is to say, when we see these sorts of things in the population at large, they are usually the result of chronic high blood pressure or underlying cardiac disease, are usually associated with a loss of the heart's pump function, and can lead to congestive heart failure, pulmonary edema, and other general badness. But in endurance athletes, who demonstrate these changes in the setting of preserved pump function, they are usually considered normal adaptations to long-term vigorous exercise that we term the athlete's heart.
What's the big deal? Aren't adaptations good?
So, in general, we think of the chronic adaptations associated with the athlete's heart to be beneficial, or at the very least neutral. They allow for us to increase our cardiac output to meet the demands of intense aerobic activity, and do not appear to be associated with the sort of pathology we would otherwise expect from these kinds of changes in heart morphology. However, there is some evidence that suggests that there may be some downside to some of the adaptations of the athlete's heart.
For example, take the dilation seen in the heart's chambers, particularly the left atrium and right ventricle. There is a hereditary disease called arrhythmogenic right ventricular cardiomyopathy, a rare condition that causes dilation of the right ventricle and fibrous deposition or "scarring" within the myocardium (the muscular layer of the heart wall). This fibrous tissue can interrupt the electrical pathways of the heart (remember that conduction system stuff?), serving as an origination point for life-threatening ventricular arrhythmias (abnormal heart rhythms). The dilated RV seen in long term athletes can be accompanied by similar fibrous deposition, leading to some speculation that there may be an "exercise-induced arrhythmogenic right ventricle" that may mimic the inherited condition. (Some have posited this as the theoretical framework for the death of Ryan Shay at the US Olympic Trials marathon in 2007, though that--in fact, all of this--remains unproven.) Dilation of the left atrium also seems to place athletes at increased risk of atrial fibrillation or atrial flutter, two abnormal heart rhythms that, while not as dangerous as ventricular arrhythmias, can still cause significant cardiovascular complications.
Another interesting cardiac finding associated with ultra-endurance exercise relates to cardiac enzymes. Many of you are probably familiar with rhabdomyolysis, a fun little problem in which repeated skeletal muscle trauma (as seen in, say, a 100-mile run) causes breakdown of muscle tissue and the release of enzymes called myoglobin and creating phosphokinase into the bloodstream. Just like skeletal muscles, heart muscle contains these enzymes; but there are also enzymes that are specific to cardiac muscle, notably troponin. Troponin is generally only minimally detectable in the bloodstream; elevated troponin levels generally imply damage to the heart muscle, usually from ischemia (lack of blood flow)-- a "heart attack." Now, several studies have detected significant elevations in troponin levels following prolonged exercise. Does this mean that we're giving ourselves small heart attacks during every ultra we run? Probably not; while troponin elevations following heart attacks tend to peak many hours after the event, and persist for several days to weeks, post-exercise troponin elevations typically appear, and resolve, very rapidly. Furthermore, while there have been studies showing reduction in LV and RV function following ultra endurance events, in almost every case function has been demonstrated to return to normal within one week, unlike what we would see in a "heart attack." It appears possible that the transient elevation in troponin following extreme exercise is related to increased permeability (leakiness) of the cardiac cell membranes rather than ischemia, cell death, or permanent heart damage.
What does all this mean?
I know, I hit you with a lot of information, and right now you might be freaking out a little bit. Freaking you out is not the objective of this post. We're going to talk big picture in a couple of weeks, and hopefully when we're done you'll feel pretty comfortable with the whole deal. For now, here's the take-home points:
Before we can get into dysfunction, though, we have to talk about normal function, and about the physiologic adaptations that the heart makes to long-term endurance exercise. Many of these adaptations are beneficial, but they're not without problems, either.
The normal heart
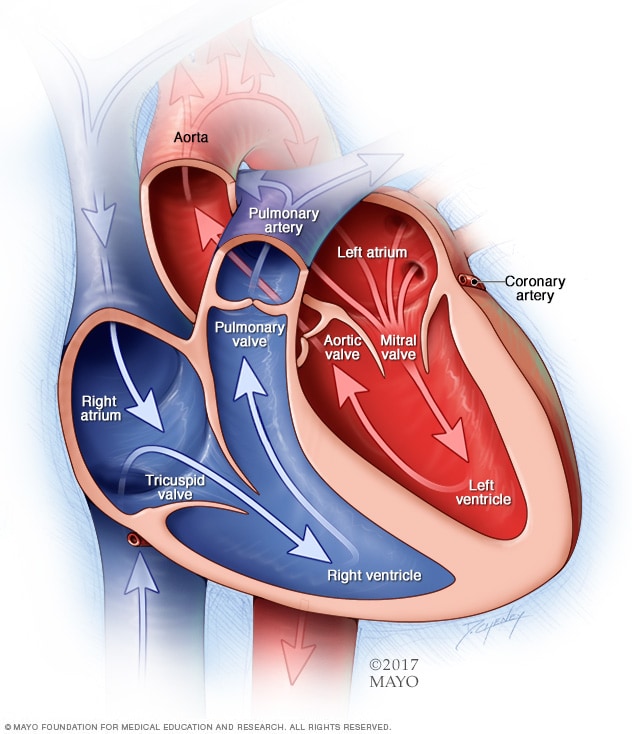 |
| Chambers (and valves) of the heart |
I don't think there's any need to get into a bunch of esoteric facts about the heart (It pumps six liters of blood per minute! It weighs 300 grams!) but we should first go through a few basics. I'm sure you can remember from ninth grade biology that the heart is a muscle that pumps blood through the body. You might also remember that the heart is split into two sides (left and right), each of which has two chambers (an atrium and a ventricle). The right side of the heart pumps de-oxygenated blood to the lungs, where the red blood cells bind to oxygen. Blood from the lungs then returns to the left side of the heart, from where (whence?) it is pumped out to the rest of the body so that the various tissues and organs can use that oxygen. Having delivered oxygen to the tissues, the blood then returns to the right side of the heart to begin the cycle again. Blood flows throughout the circulatory system in what is essentially a series of tubes; veins carry blood to the heart, while arteries carry blood from the heart.
OK, simple enough. From a basic standpoint, that's all we need the heart to do: pump oxygen-poor blood to the lungs, deliver oxygen-rich blood to the rest of the body. So when we talk about cardiac disease, we're most generally talking about a failure of the heart to fulfill that function. But there are a bunch of different ways in which this basic function can be compromised. For our purposes, there are three systems inherent to normal heart function that we want to be familiar with in order to understand possible dysfunction: the coronary arteries, the conduction system, and the heart muscle itself.
 |
| Coronary arteries |
We spoke briefly about the heart muscle last week; simply put, the muscle squeezes, increasing the pressure within the chambers of the heart, and forces blood out into the circulation. The muscle is the heart's engine. The coronary arteries are responsible for delivering oxygen to the heart muscle. Wait a minute, you're saying, didn't you just say that arteries carry blood AWAY from the heart? Yes, I did! Thanks for paying attention! Arteries do indeed carry blood away from the ventricles, but in this case they don't have to go very far. The coronary arteries arise from the aorta immediately after the blood leaves the left ventricle, and they surround the heart, supplying oxygen-rich blood to the muscle. When you hear the term "heart attack," this is usually used to mean an interruption of blood flow to the heart muscle, usually due to a narrowing of, or blockage within, the coronary arteries. We're going to do an entire post about the coronary arteries next week, so for now, just think of them as the heart's plumbing system.
The conduction system, then, is the wiring. This system is comprised of electrical fibers that coordinate the heartbeat. The depolarization of these electrical cells causes the atria, and then the ventricles, to contract synchronously. The contraction of the atria forces blood into the ventricles, and the contraction of the ventricles forces blood out into the circulation. When you see that familiar tracing that we all know represents a beating heart:
what you're looking at is a graphic representation of the heart's electrical activity. (I'm not going to go into what each of those little squiggles means, but if you're interested, read this.) Without the orderly input of the electrical/conduction system, these contractions may lose their synchronicity, robbing the heart muscle of its ability to pump blood effectively--or contractions can cease altogether.
The athlete's heart
 |
| Note the enlarged (dilated) cardiac chambers in the athlete's vs. non-athlete's heart. Photo: cyclingtips.com |
What's the big deal? Aren't adaptations good?
So, in general, we think of the chronic adaptations associated with the athlete's heart to be beneficial, or at the very least neutral. They allow for us to increase our cardiac output to meet the demands of intense aerobic activity, and do not appear to be associated with the sort of pathology we would otherwise expect from these kinds of changes in heart morphology. However, there is some evidence that suggests that there may be some downside to some of the adaptations of the athlete's heart.
For example, take the dilation seen in the heart's chambers, particularly the left atrium and right ventricle. There is a hereditary disease called arrhythmogenic right ventricular cardiomyopathy, a rare condition that causes dilation of the right ventricle and fibrous deposition or "scarring" within the myocardium (the muscular layer of the heart wall). This fibrous tissue can interrupt the electrical pathways of the heart (remember that conduction system stuff?), serving as an origination point for life-threatening ventricular arrhythmias (abnormal heart rhythms). The dilated RV seen in long term athletes can be accompanied by similar fibrous deposition, leading to some speculation that there may be an "exercise-induced arrhythmogenic right ventricle" that may mimic the inherited condition. (Some have posited this as the theoretical framework for the death of Ryan Shay at the US Olympic Trials marathon in 2007, though that--in fact, all of this--remains unproven.) Dilation of the left atrium also seems to place athletes at increased risk of atrial fibrillation or atrial flutter, two abnormal heart rhythms that, while not as dangerous as ventricular arrhythmias, can still cause significant cardiovascular complications.
 |
| No bueno. |
Another interesting cardiac finding associated with ultra-endurance exercise relates to cardiac enzymes. Many of you are probably familiar with rhabdomyolysis, a fun little problem in which repeated skeletal muscle trauma (as seen in, say, a 100-mile run) causes breakdown of muscle tissue and the release of enzymes called myoglobin and creating phosphokinase into the bloodstream. Just like skeletal muscles, heart muscle contains these enzymes; but there are also enzymes that are specific to cardiac muscle, notably troponin. Troponin is generally only minimally detectable in the bloodstream; elevated troponin levels generally imply damage to the heart muscle, usually from ischemia (lack of blood flow)-- a "heart attack." Now, several studies have detected significant elevations in troponin levels following prolonged exercise. Does this mean that we're giving ourselves small heart attacks during every ultra we run? Probably not; while troponin elevations following heart attacks tend to peak many hours after the event, and persist for several days to weeks, post-exercise troponin elevations typically appear, and resolve, very rapidly. Furthermore, while there have been studies showing reduction in LV and RV function following ultra endurance events, in almost every case function has been demonstrated to return to normal within one week, unlike what we would see in a "heart attack." It appears possible that the transient elevation in troponin following extreme exercise is related to increased permeability (leakiness) of the cardiac cell membranes rather than ischemia, cell death, or permanent heart damage.
What does all this mean?
I know, I hit you with a lot of information, and right now you might be freaking out a little bit. Freaking you out is not the objective of this post. We're going to talk big picture in a couple of weeks, and hopefully when we're done you'll feel pretty comfortable with the whole deal. For now, here's the take-home points:
- there are several adaptations that the heart makes to accommodate long-term, vigorous aerobic exercise
- most of these adaptations are generally beneficial
- there are some morphologic changes (that is, the the size/shape of the heart) that may increase the risk of arrhythmias in athletes
- most of the evidence we have at this time shows correlation, not causation, and much of the framework surrounding this remains theoretical/speculative
Again, we'll go big picture in a couple of weeks, and I'll be able to draw things together a little bit more. The point of all this is just to make you a little more aware and informed about some of the interesting stuff that's out there, and maybe to generate some fodder for a discussion with your doctor if you have questions or concerns.
If you want some really detailed reading on this stuff, check our these highly scientific articles:
